Copper (Cu) Periodic Table (Element Information & More)
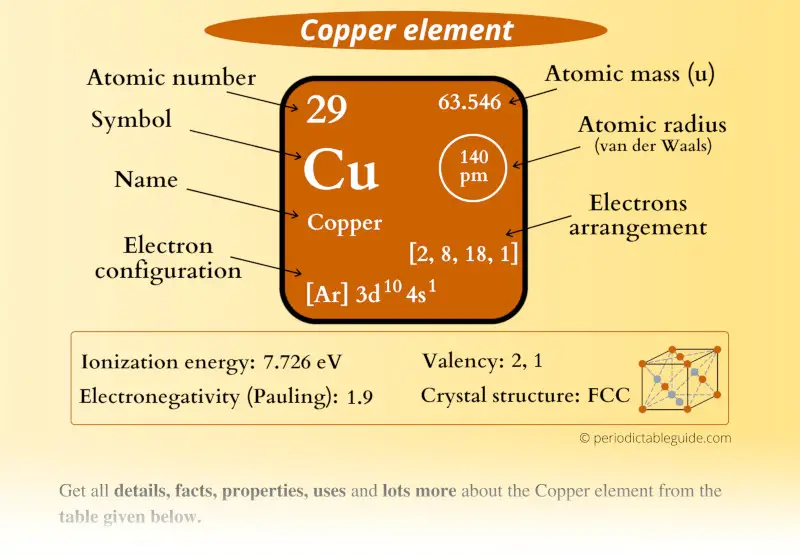
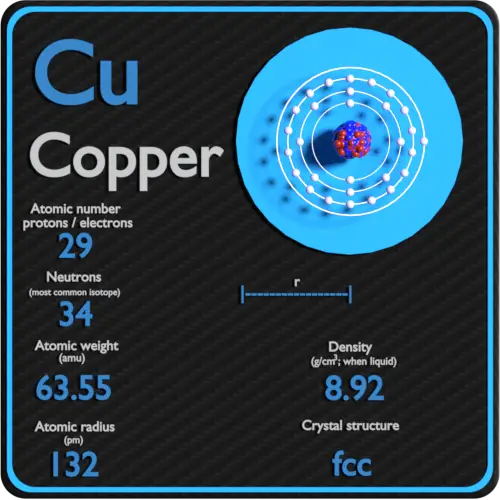
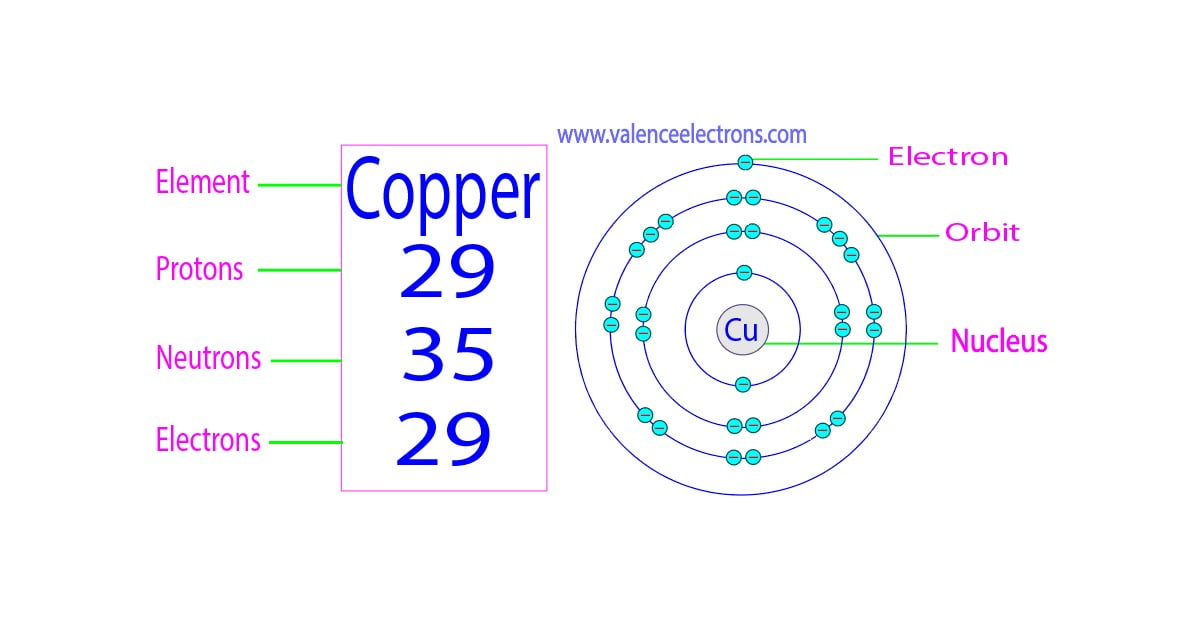
Number of Protons: 29: Number of Neutrons: 35: Number of Electrons: 29: Melting Point: 1083.0° C: Boiling Point: 2567.0° C: Density: 8.96 grams per cubic centimeter. Copper beads dating back to 9000 B.C. were found in Iraq: Common Compounds: Copper chloride (CuCl 2) Copper cyanide (CuCN) - electroplating; Cuprous chloride (CuCl) - absorbs.
Copper Periodic Table and Atomic Properties
For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom. Number of Electrons in Copper = Atomic number of Copper = 29. I hope you have understood the simple method for finding the protons, neutrons and electrons of copper atom. Check out related topics for more practice; Zinc protons neutrons electrons.

Copper Periodic Table Protons And Neutrons Review Home Decor
In this video we'll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Copper (Cu). From the Periodic.

Copper Periodic Table Protons Neutrons And Electrons Awesome Home
Atoms of the same element have the same number of protons, and atoms of different elements have different numbers of protons. In other words, the number of protons in an atom defines the element.. and copper-65 (64.9 amu), in abundances 69% + 31%. The standard atomic weight for copper is the average, weighted by their natural abundance.
How many protons, neutrons and electrons does copper have? (2023)
Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content .. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted.
How to Find the Valence Electrons for Copper (Cu)?
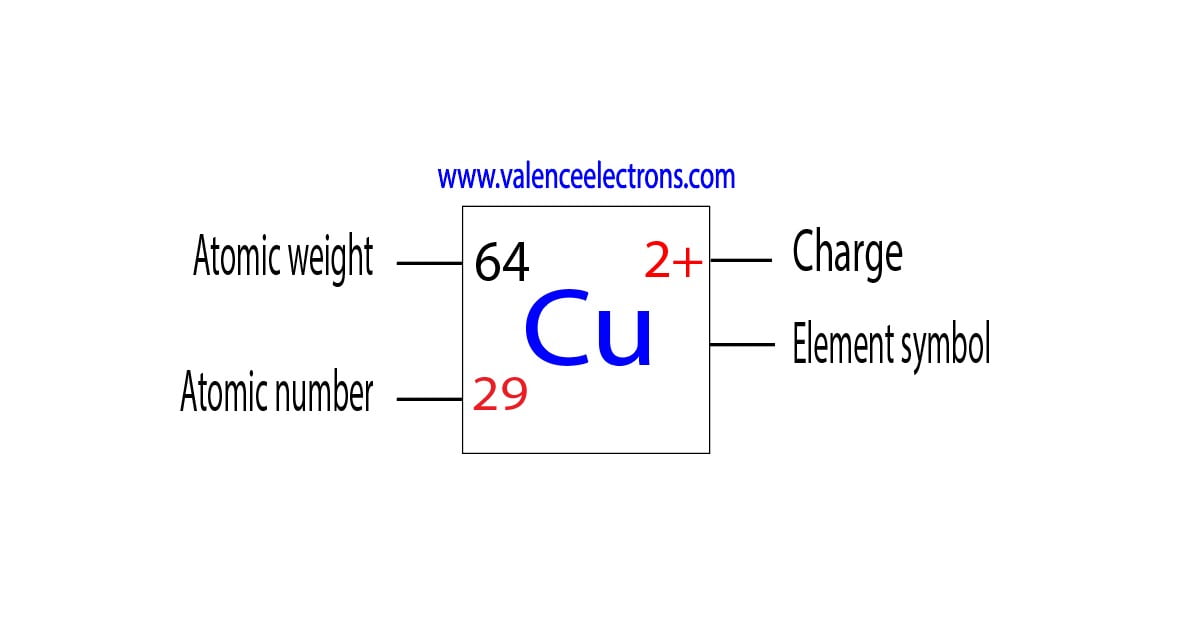
Atomic Number of Copper is 29. Chemical symbol for Copper is Cu. Number of protons in Copper is 29. Atomic weight of Copper is 63.546 u or g/mol. Melting point of Copper is 1083,5 °C and its the boiling point is 2595 °C. » Boiling Point » Melting Point » Abundant » State at STP » Discovery Year.

How to Find the Valence Electrons for Copper (Cu)?
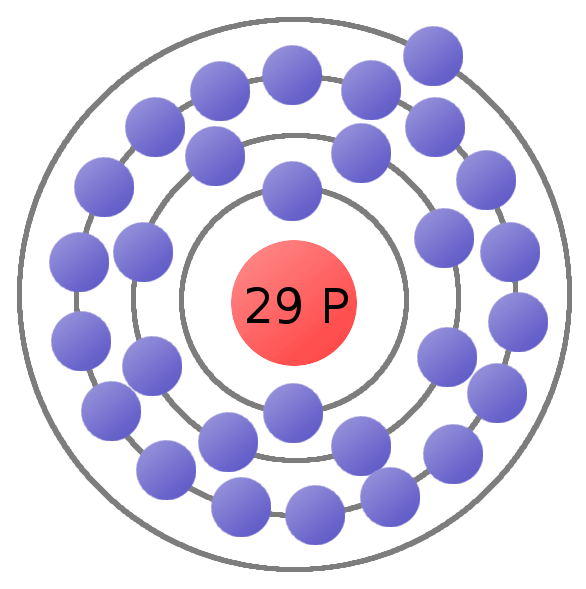
Atomic Number - Protons, Electrons and Neutrons in Copper. Copper is a chemical element with atomic number 29 which means there are 29 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Copper Periodic Table Protons Neutrons And Electrons Bios Pics
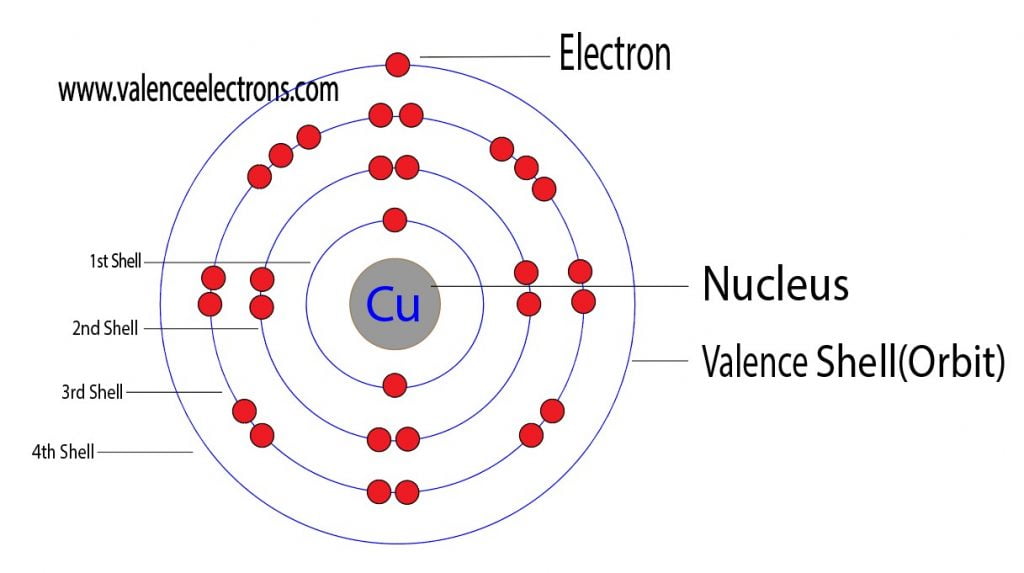
There are 29 protons, 35 neutrons, and 29 electrons in a copper atom. You can find these numbers yourself by looking at a periodic table of the elements: Copper will be the 29th element in the table reading from left to right, top to bottom. The box for copper will look something like this: The number in the top-left corner is called the atomic.
Symbol and electron diagram for Copper illustration Stock Vector Image & Art Alamy
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise. An ion of platinum has a mass number of 195 and contains 74 electrons.

Solar Energy Introduction Course
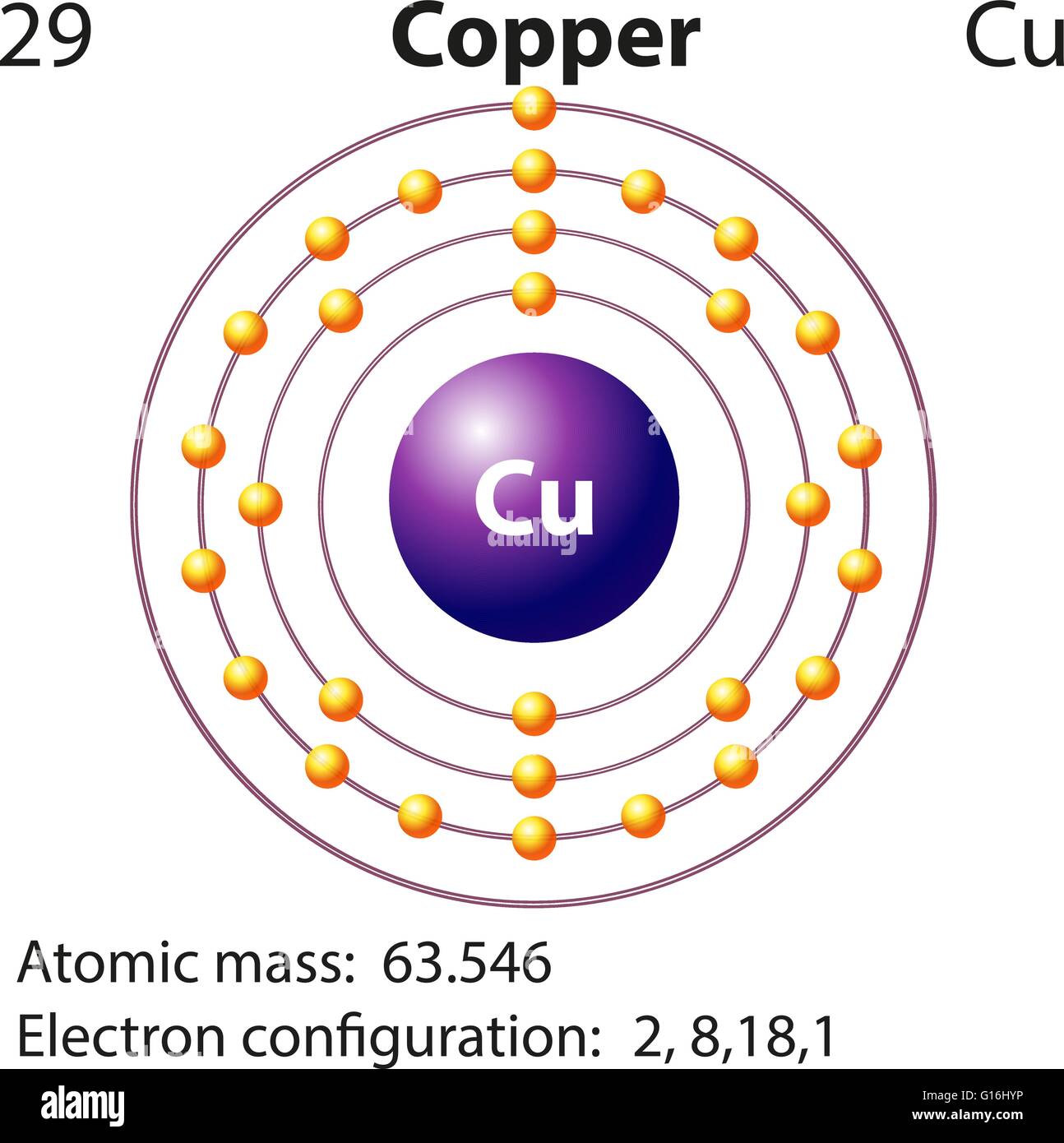
Copper is a chemical element of the periodic table with chemical symbol Cu and atomic number 29 with an atomic weight of 63.5463 u and is classed as a transition metal.. Number of protons: 29 p + Number of neutrons: 35 n 0: Number of electrons: 29 e-From Wikipedia,.

Copper Periodic Table Protons Neutrons And Electrons Awesome Home
In this case, the copper atom carries a positive charge. Cu - e - → Cu +. Here, the electron configuration of copper ion (Cu +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This copper ion (Cu +) has twenty-nine protons, thirty-five neutrons, and twenty-eight electrons. Also, copper has one more ion.

PPT Internal Structure of Atoms PowerPoint Presentation, free download ID9683214
The number of protons in the nucleus is given by the atomic number, \(Z\). The number of neutrons in the nucleus is the neutron number, \(N\). The total number of nucleons is the mass number, \(A\).. we must take special care when quoting the mass of an element. For example, Copper (Cu) has two stable isotopes: \[_{29}^{63} Cu ( 62.929595.
Copper Protons Neutrons Electrons Electron Configuration
Protons and Neutrons in Copper. Copper is a chemical element with atomic number 29 which means there are 29 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

How to find Protons & Electrons for Cu+ and Cu2+ (Copper II and III ions) YouTube
Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.

Electron Configuration Of Copper 1+ worksheet
Number of protons = number of electrons = atomic number. Number of neutrons = mass number - atomic number. Key fact. Remember that Protons are Positive, and Neutrons are Neutral. Question.
💐 Copper atom model. Copper Particles. 20221017
The dosimeters must be capable of accurately quantifying the number of protons during therapy in real time, ideally at the single-proton level. However, because ionization chambers and silicon.